An Evaluation of The Effects of Glabridin, Dexamethasone, and Iberiotoxin on Liver Injury in A Rat Model of Bleomycin-Induced Pulmonary Fibrosis
DOI:
https://doi.org/10.5281/zenodo.16987253Keywords:
Bleomycin, Dexamethasone, Glabridin, Iberiotoxin, Liver fibrosisAbstract
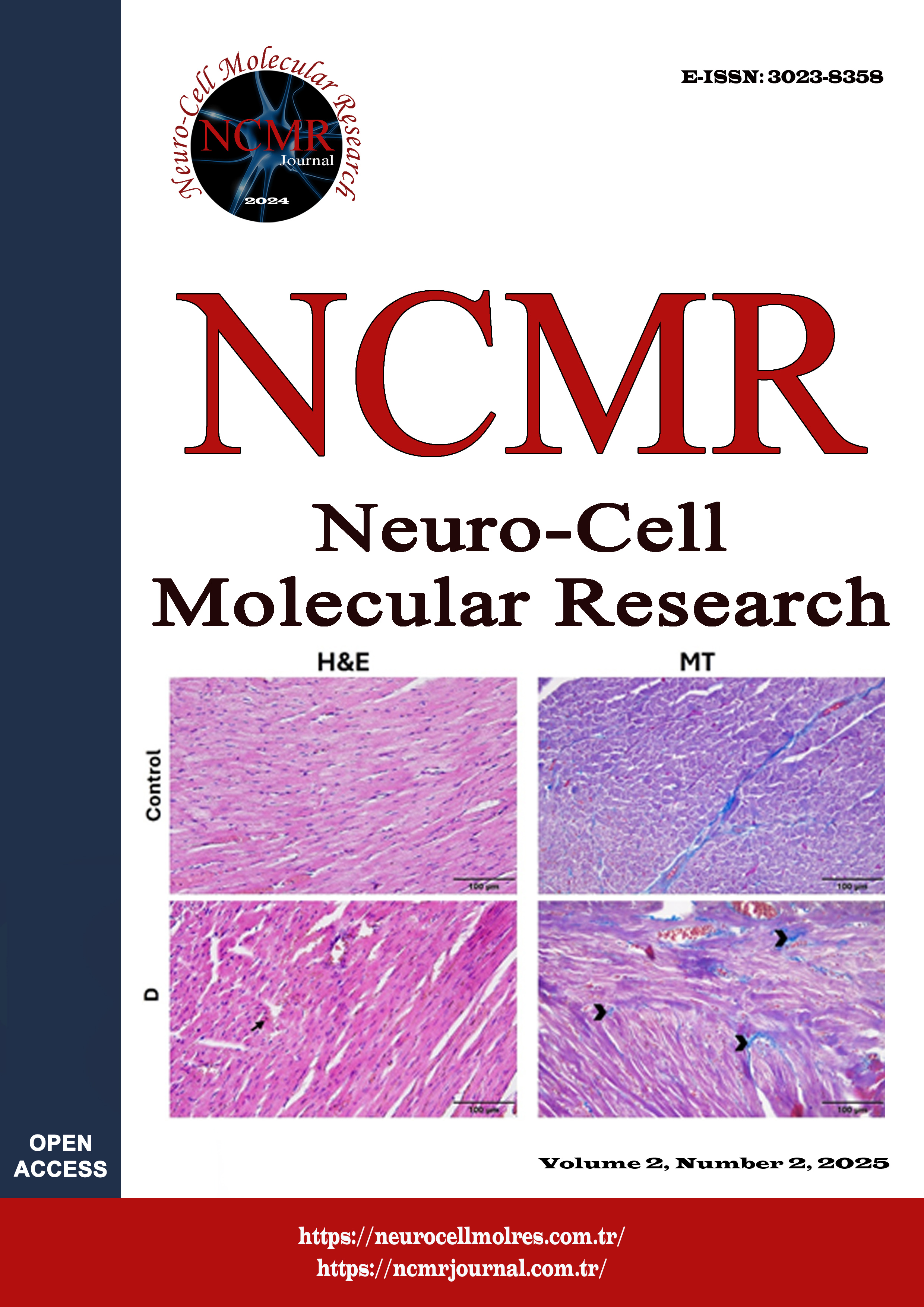
This study investigated the potential protective effects of glabridin (Gla), dexamethasone (DEX), and iberiotoxin (Ibx), a BKCa channel inhibitor, against liver damage in a bleomycin (BLM)-induced pulmonary fibrosis model. Thirty-six female Wistar Albino rats were randomly divided into six equal groups. Rats in the Sham group received 0.1 ml physiological saline intratracheally. Rats in the BLM group were administered 0.1 ml of BLM (5 mg/kg) intratracheally to induce pulmonary fibrosis. In the treatment groups with induced pulmonary fibrosis, the BLM+Gla group received 30 mg/kg Gla dissolved in 20% dimethyl sulfoxide (DMSO) intraperitoneally (i.p.), the BLM+Dex group 1 mg/kg Dex i.p. following the same dosing schedule, and the BLM+Ibx+Gla group rats 6 µg/kg Ibx i.p. 30 minutes before 30 mg/kg Gla. At the end of the experiment, liver tissues were evaluated histopathologically using hematoxylin-eosin (H&E) staining. Significant histopathological alterations were observed in the liver tissues from the BLM, BLM+Dex, and BLM+Vehicle groups, including disorganized hepatocyte architecture, loss of cellular boundaries, increased numbers of degenerative cells, and marked infiltration of inflammatory cells. In contrast, liver tissues from the BLM+Gla and BLM+Ibx+Gla groups largely maintained a normal histological architecture. These groups exhibited a reduced number of degenerative cells, preservation of acidophilic cytoplasmic characteristics, and a connective tissue density comparable to that of the sham group. The findings of this study suggest that BLM-induced pulmonary fibrosis may lead to secondary liver injury characterized by histopathological alterations and fibrotic changes. Gla demonstrated notable hepatoprotective effects by preserving liver architecture and reducing tissue damage, while Dex exhibited only partial efficacy. Furthermore, Gla combined with Ibx enhanced protective outcomes, highlighting its potential therapeutic value. These results provide new insights into the systemic consequences of pulmonary fibrosis and support the exploration of Gla-based strategies for managing hepatic fibrosis.
Downloads
References
Richter K, Konzack A, Pihlajaniemi T, Heljasvaara R, Kietzmann T. Redox-fibrosis: Impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol. 2015;6:344–52. https://doi.org/10.1016/j.redox.2015.08.015
Agrawal A, Verma I, Shah V, Agarwal A, Sikachi RR. Cardiac manifestations of idiopathic pulmonary fibrosis. Intractable Rare Dis Res. 2016;5(2):70–5. https://doi.org/10.5582/irdr.2016.01023
Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res. 201;97:122–30. https://doi.org/10.1016/j.phrs.2015.04.012
Cocconcelli E, Tonelli R, Abbati G, Marchioni A, Castaniere I, Pelizzaro F, Russo FP, Vegetti A, Balestro E, Pietrangelo A, Richeldi L, Luppi F, Spagnolo P, Clini E, Cerri S. Subclinical liver fibrosis in patients with idiopathic pulmonary fibrosis. Intern Emerg Med. 2021;16(2):349–57. https://doi.org/10.1007/s11739-020-02376-2
Vásquez-Garzón VR, Ramírez-Cosmes A, Reyes-Jiménez E, Carrasco-Torres G, Hernández-García S, Aguilar-Ruiz SR, Torres-Aguilar H, Alpuche J, Pérez-Campos Mayoral L, Pina-Canseco S, Arellanes-Robledo J, Villa-Treviño S, Baltiérrez-Hoyos R. Liver damage in bleomycin-induced pulmonary fibrosis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(12):1503–13. https://doi.org/10.1007/s00210-019-01690-7